AG — Prof. Heiko Rieger — Statistical Physics
Biological Physics
Biological systems are manifestly far from equilibrium, since they consume continuously energy for instance in the form of ATP. In collaboration with several groups from the life science departments of the Saarland University and within the collaborative research center SFB 1027 we work on the biophysics of killing: and study how cytotoxic killer cells of the human body eliminate pathogen-infected or tumorigenic target cells. We are also interested in understanding the physical determinants of tumor growth, in particular the process of vascularization, interstitial fluid flow and drug delivery.
Current Research:
Optimal non-Markovian search strategies with n-step memory
Stochastic search processes are ubiquitous in nature and are expected to become more efficient when equipped with a memory, where the searcher has been before. A natural realization of a search process with long-lasting memory is a migrating cell that is repelled from the diffusive chemotactic signal that it secretes on its way, denoted as an autochemotactic searcher. To analyze the efficiency of this class of non Markovian search processes, we present a general formalism that allows one to compute the mean first passage time (MFPT) for a given set of conditional transition probabilities for non-Markovian random walks on a lattice. We show that the optimal choice of the n-step transition probabilities decreases the MFPT systematically and substantially with an increasing number of steps. It turns out that the optimal search strategies can be reduced to simple cycles defined by a small parameter set and that mirrorasymmetric walks are more efficient. For the autochemotactic searcher, we show that an optimal coupling between the searcher and the chemical reduces the MFPT to 1/3 of the one for a Markovian random walk.
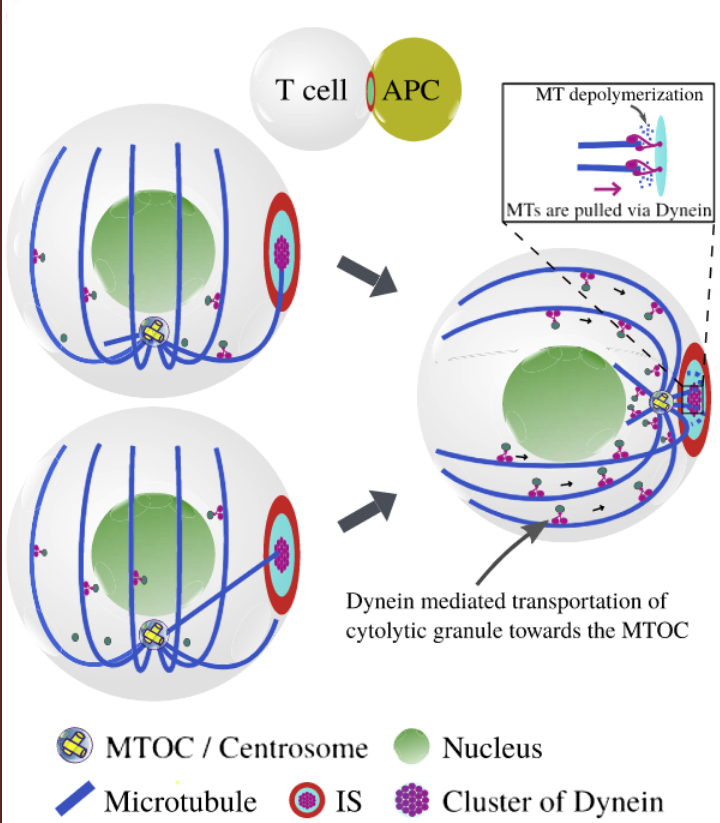
Stochastic model of T Cell repolarization during target elimination
Cytotoxic T lymphocytes (T cells) and natural killer cells form a tight contact, the immunological synapse (IS), with target cells, where they release their lytic granules containing perforin/granzyme and cytokine containing vesicles. During this process the cell repolarizes and moves the microtubule organizing center (MTOC) towards the IS. In the first part of our work we developed a computational model for the molecular-motor-driven motion of the MT cytoskeleton confined between plasma membrane and nucleus during T cell polarization and analyzed different mechanisms (cortical sliding and capture-shrinkage) that have been proposed on the basis of recent experiments. Here we use this model to analyze the dynamics of the MTOC during the repositioning process in situations in which a) the IS is in an arbitrary position with respect to the initial position of the MTOC and b) the T cell has two IS at two arbitrary positions. We observe several scenarios that have also been reported experimentally: the MTOC alternates stochastically (but with a well defined average transition time) between the two IS; it wiggles in between the two IS without transiting to one of the two; or it is at some point pulled to one of the two IS and stays there. Our model allows to predict which scenario emerges in dependency of the mechanisms in action and the number of dyneins present.
Migration of Cytotoxic T Lymphocytes in 3D Collagen Matrices
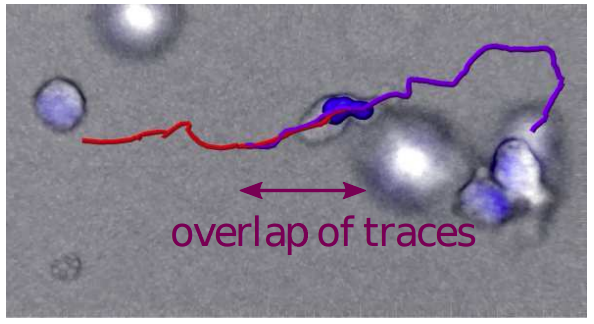
CD8+ cytotoxic T lymphocytes (CTL) and natural killer (NK) cells are the main cytotoxic killer cells of the human body to eliminate pathogen-infected or tumorigenic cells (= target cells). To find their targets they have to navigate and migrate through a complex biological microenvironments, a key component of which is the extracellular matrix (ECM). The mechanisms underlying killer cell’s navigation are not well understood. To mimic an ECM we use a matrix formed by different collagen concentrations, and analyze migration trajectories of primary human CTLs. Different migration patterns are observed and can be grouped into three motility types: slow, fast and mixed. The dynamics are well described by a two-state persistent random walk model which allows cells to switch between slow motion with low persistence, and fast motion with high persistence. We hypothesize that the slow motility mode describes CTLs creating channels through the collagen matrix by deforming and tearing apart collagen fibers, and that the fast motility mode describes CTLs moving within these channels. Experimental evidence supporting this scenario is presented by visualizing migrating T cells following each other on exactly the same track and showing cells moving quickly in channel-like cavities within the surrounding collagen matrix. Consequently, the efficiency of the stochastic search process of CTLs in the ECM should strongly be influenced by a dynamically changing channel network produced by the killer cells themselves.
Interorganelle Tethering to Endocytic Organelles Determines Directional Cytokine Transport in CD4+ T Cells
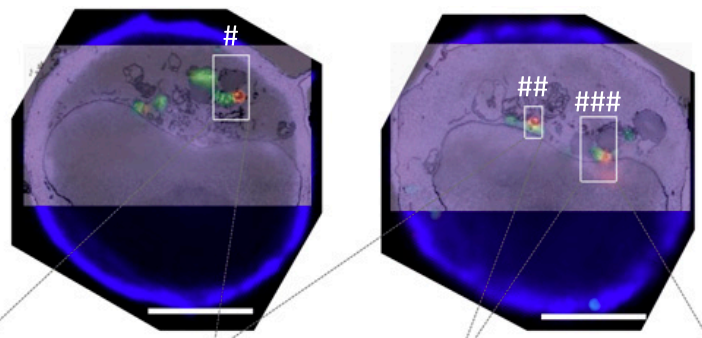
Delivery of vesicles to their desired destinations plays a central role in maintaining proper cell functionality. In certain scenarios, depending on loaded cargos, the vesicles have spatially distinct destinations. For example, in T cells, some cytokines (e.g., IL-2) are polarized to the T cell–target cell interface, whereas the other cytokines are delivered multidirectionally (e.g., TNF-a). In this study, we show that in primary human CD4+ T cells, both TNF-α+ and IL-2+ vesicles can tether with endocytic organelles (lysosomes/late endosomes) by forming membrane contact sites. Tethered cytokine-containing vesicle (CytV)–endocytic organelle pairs are released sequentially. Only endocytic organelle-tethered CytVs are preferentially transported to their desired destination. Mathematical models suggest that endocytic organelle tethering could regulate the direction of cytokine transport by selectively attaching different microtubule motor proteins (such as kinesin and dynein) to the corresponding CytVs. These findings establish the previously unknown interorganelle tethering to endocytic organelles as a universal solution for directional cytokine transport in CD4+ T cells. Modulating tethering to endocytic organelles can, therefore, coordinately control directionally distinct cytokine transport.
Oxygen in the Tumor Microenvironment: Mathematical and Numerical Modeling
There are many reasons to try to achieve a good grasp of the distribution of oxygen in the tumor microenvironment. The lack of oxygen – hypoxia – is a main actor in the evolution of tumors and in their growth and appears to be just as important in tumor invasion and metastasis. Mathematical models of the distribution of oxygen in tumors which are based on reaction-diffusion equations provide partial but qualitatively significant descriptions of the measured oxygen concentrations in the tumor microenvironment, especially when they incorporate important elements of the blood vessel network such as the blood vessel size and spatial distribution and the pulsation of local pressure due to blood circulation. Here, we review our mathematical and numerical approaches to the distribution of oxygen that yield insights both on the role of the distribution of blood vessel density and size and on the fluctuations of blood pressure.
Computational models for active matter
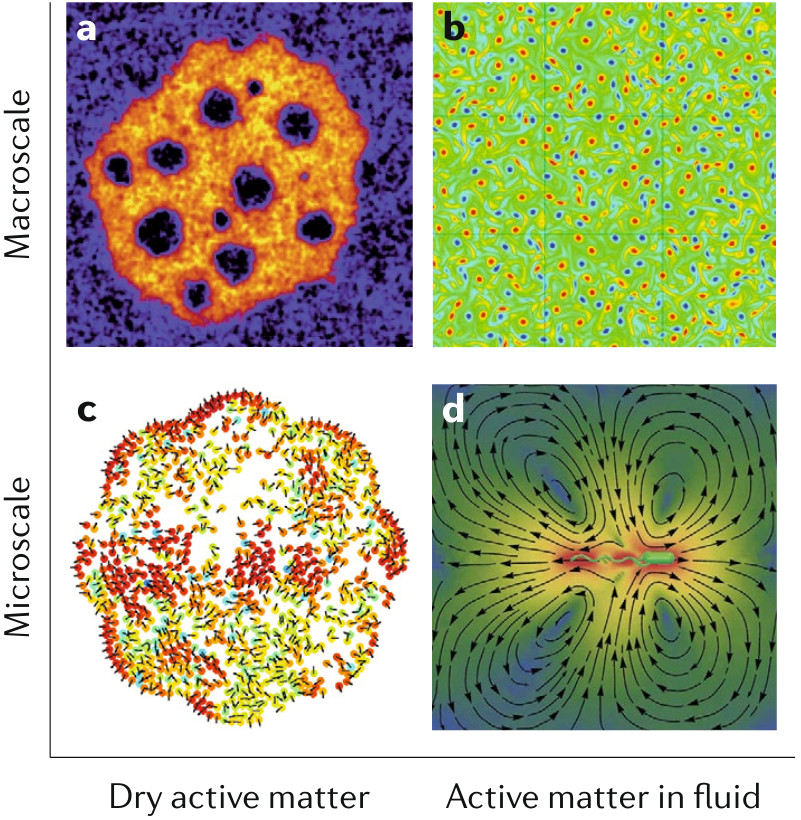
Active matter, which ranges from molecular motors to groups of animals, exists at different length scales and timescales, and various computational models have been proposed to describe and predict its behaviour. The diversity of the methods and the challenges in modelling active matter primarily originate from the out- of-equilibrium character, lack of detailed balance and of time- reversal symmetry , multiscale nature, nonlinearity and multibody interactions. Models exist for both dry active matter and active matter in fluids, and can be agent- based or continuum-level descriptions. They can be generic, emphasizing universal features, or detailed, capturing specific features. We compare various modelling approaches and numerical techniques to illuminate the innovations and challenges in understanding active matter.
Capillary action in scalar active matter

We study the capacity of active matter to rise in thin tubes against gravity and other related phenomena, like, wetting of vertical plates and spontaneous imbibition, where a wetting liquid is drawn into a porous medium. This capillary action or capillarity is well known in classical fluids and originates from attractive interactions between the liquid molecules and the container walls, and from the attraction of the liquid molecules among each other. We observe capillarity in a minimal model for scalar active matter with purely repulsive interactions, where an effective attraction emerges due to slowdown during collisions between active particles and between active particles and walls. Simulations indicate that the capillary rise in thin tubes is approximately proportional to the active sedimentation length λ and that the wetting height of a vertical plate grows superlinear with λ. In a disordered porous medium the imbibition height scales as <h> ∝ λΦm, where Φm is its packing fraction.
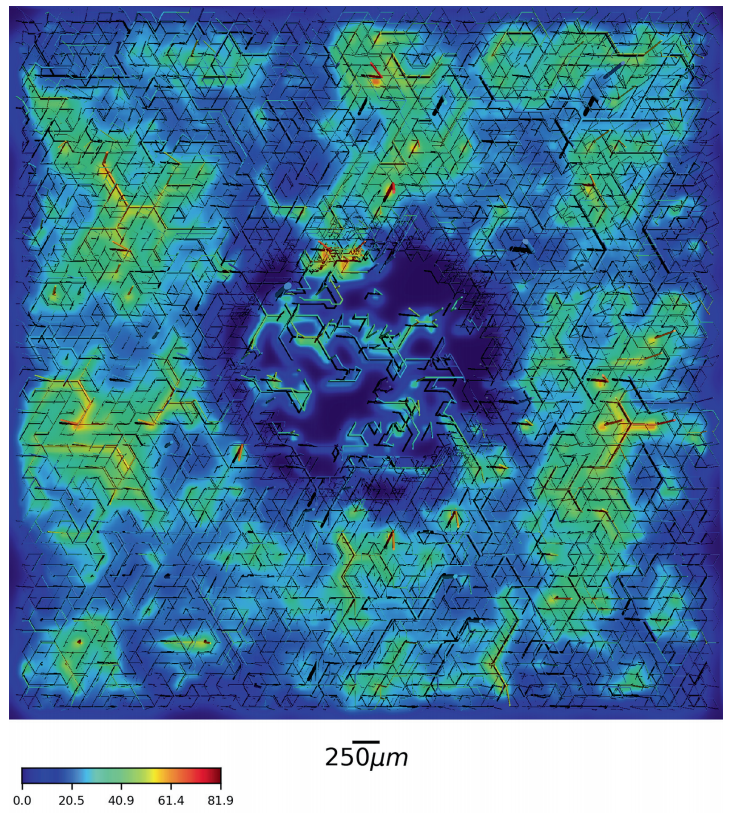
Dynamic vessel adaptation in synthetic arteriovenous networks
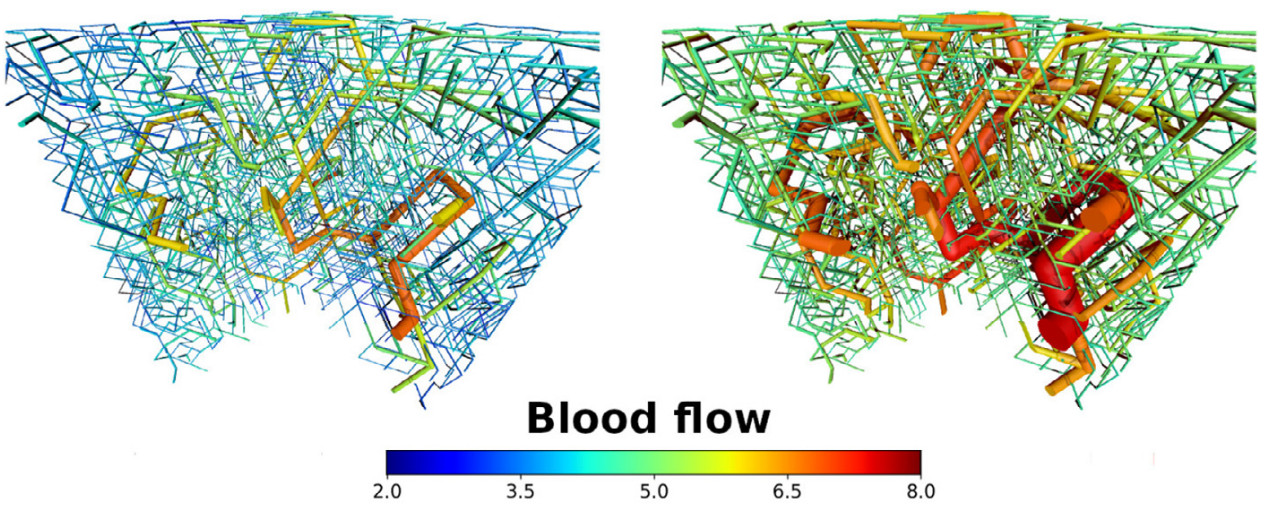
Blood vessel networks of living organisms continuously adapt their structure under the influence of hemodynamic and metabolic stimuli. For a fixed vessel arrangement, blood flow characteristics still depend crucially on the morphology of each vessel. Vessel diameters adapt dynamically according to internal and external stimuli: Endothelial wall shear stress, intravascular pressure, flow-dependent metabolic stimuli, and electrical stimuli conducted from distal to proximal segments along vascular walls. Pries et al. formulated a theoretical model involving these four local stimuli to simulate long-term changes of vessel diameters during structural adaption of microvascular networks. Here we apply this vessel adaptation algorithm to synthetic arteriovenous blood vessel networks generated by our simulation framework "Tumorcode". We fixed the free model parameters by an optimization method combined with the requirement of homogeneous flow in the capillary bed. We find that the local blood volume, surface to volume ratio and branching ratio differs from networks with radii fulfilling Murray's law exactly to networks with radii obtained by the adaptation algorithm although their relation is close to Murray's law.
Fine-grained simulations of the microenvironment of vascularized tumours
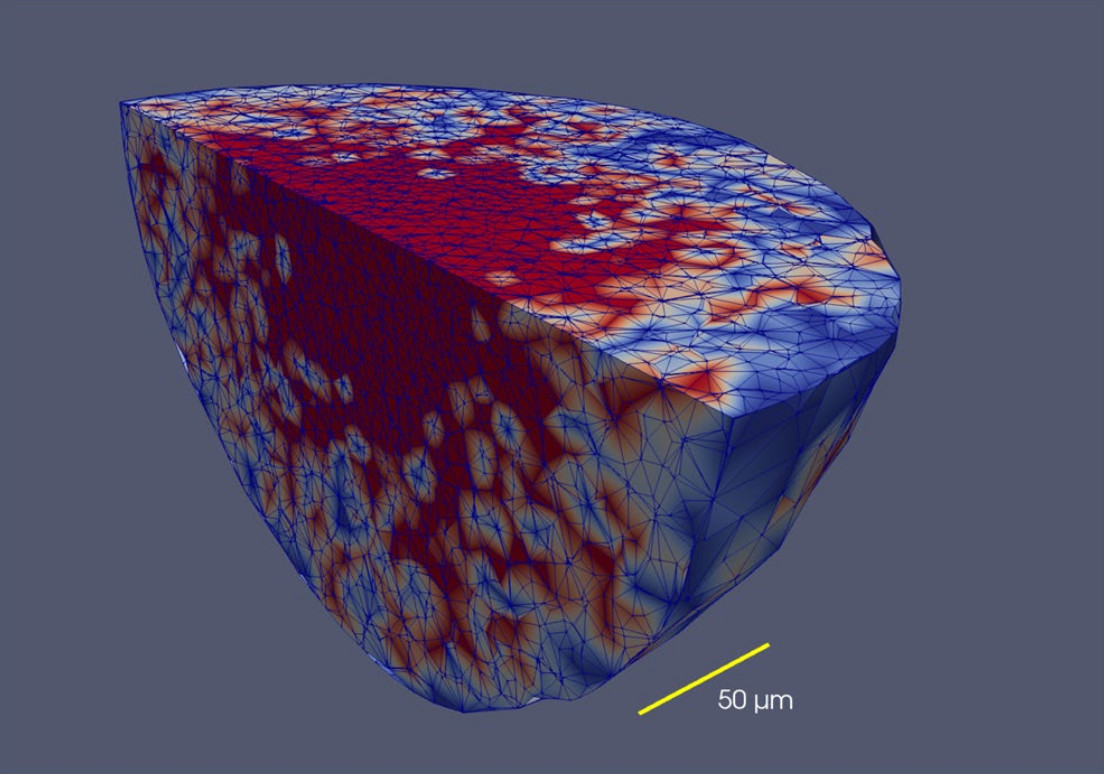
One of many important features of the tumour microenvironment is that it is a place of active Darwinian selection where different tumour clones become adapted to the variety of ecological niches that make up the microenvironment. these evolutionary processes turn the microenvironment into a powerful source of tumour heterogeneity and contribute to the development of drug resistance in cancer. Here, we describe a computational tool to study the ecology of the microenvironment and report results about the ecology of the tumour microenvironment and its evolutionary dynamics.
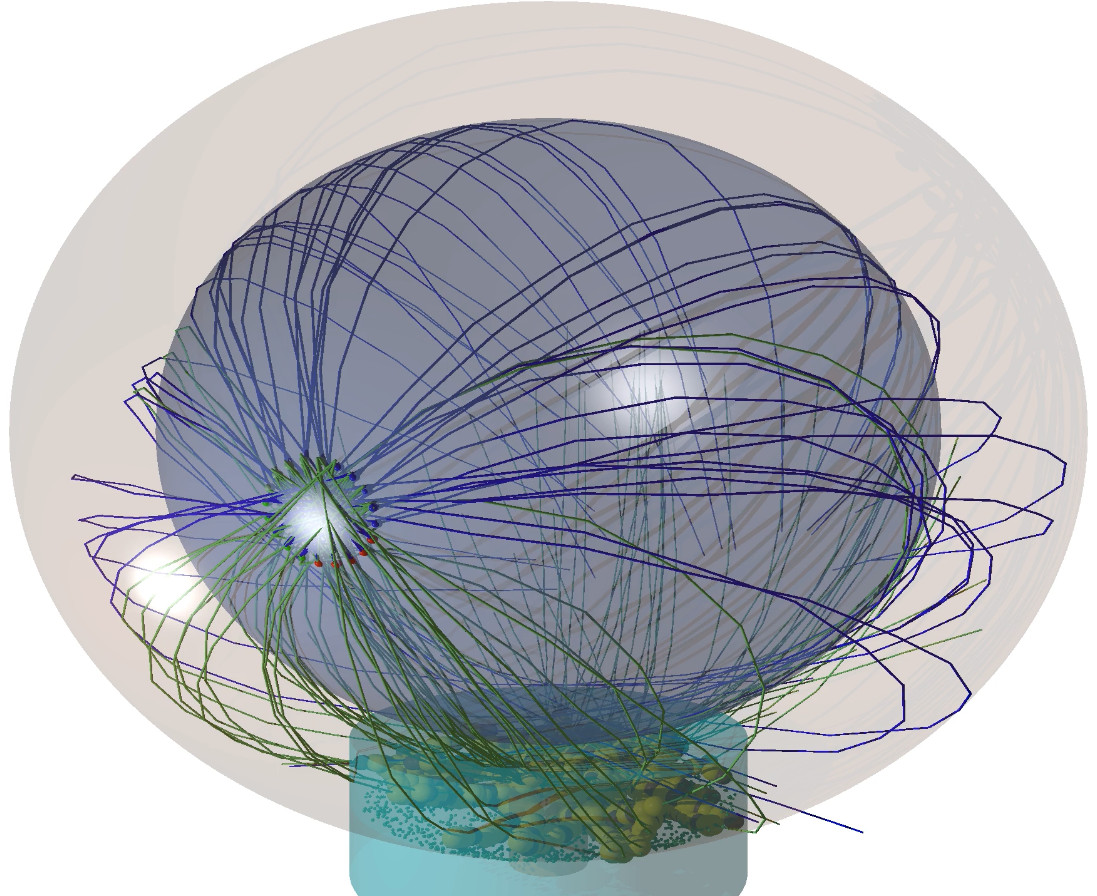
Search and capture efficiency of dynamic microtubules for centrosome relocation during IS formation
Upon contact with antigen-presenting cells, cytotoxic T lymphocytes (T cells) establish a highly organized contact zone denoted as the immunological synapse (IS). The formation of the IS implies relocation of the microtubule organizing center (MTOC) toward the contact zone, which necessitates a proper connection between the MTOC and the IS via dynamic microtubules (MTs). The efficiency of the MTs finding the IS within the relevant timescale is, however, still illusive. We investigate how MTs search the three-dimensional constrained cellular volume for the IS and bind upon encounter to dynein anchored at the IS cortex. The search efficiency is estimated by calculating the time required for the MTs to reach the dynein-enriched region of the IS. In this study, we develop simple mathematical and numerical models incorporating relevant components of a cell and propose an optimal search strategy. Using the mathematical model, we have quantified the average search time for a wide range of model parameters and proposed an optimized set of values leading to the minimal capture time. Our results show that search times are minimal when the IS formed at the nearest or at the farthest sites on the cell surface with respect to the perinuclear MTOC. The search time increases monotonically away from these two specific sites and is maximal at an intermediate position near the equator of the cell. We observed that search time strongly depends on the number of searching MTs and distance of the MTOC from the nuclear surface.
Optimality of spatially inhomogeneous search strategies
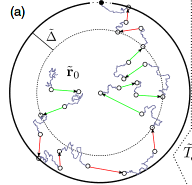
We consider intermittent search processes, which alternate stochastically between slow, diffusive motion in which the target can be detected and fast ballistic motion during which targets cannot be detected. The distribution function of ballistic motion directions varies from point to point in space. The specific space dependence of this distribution together with the switching rates between the two modes of motion establishes a spatially inhomogeneous search strategy. We show that the mean first passage times for several standard search problems -- narrow escape, reaction partner finding, reaction-escape -- can be minimized with a directional distribution that is reminiscent of the spatial organization of the cytoskeleton filaments of cells with a centrosome: radial ballistic transport from center to periphery and back, and ballistic transport in random directions within a concentric shell of thickness Δopt along the domain boundary.
Reaction-diffusion model for STIM-ORAI interaction: the role of ROS and mutations

Release of Ca2+ from endoplasmatic retriculum (ER) Ca2+ stores causes stromal interaction molecules (STIM) in the ER membrane and ORAI proteins in the plasma membrane (PM) to interact and form the Ca2+ release activated Ca2+ (CRAC) channels, which represent a major Ca2+ entry route in non-excitable cells and thus control various cell functions. It is experimentally possible to mutate ORAI1 proteins and therefore modify, especially block, the Ca2+ influx into the cell. On the basis of the model of Hoover and Lewis (2011), we formulate a reaction-diffusion model to quantify the STIM1-ORAI1 interaction during CRAC channel formation and analyze different ORAI1 channel stoichiometries and different ratios of STIM1 and ORAI1 in comparison with experimental data. We incorporate the inhibition of ORAI1 channels by ROS into our model and calculate its contribution to the CRAC channel amplitude. We observe a large decrease of the CRAC channel amplitude evoked by mutations of ORAI1 proteins.
Spatial cytoskeleton organization supports targeted intracellular transport
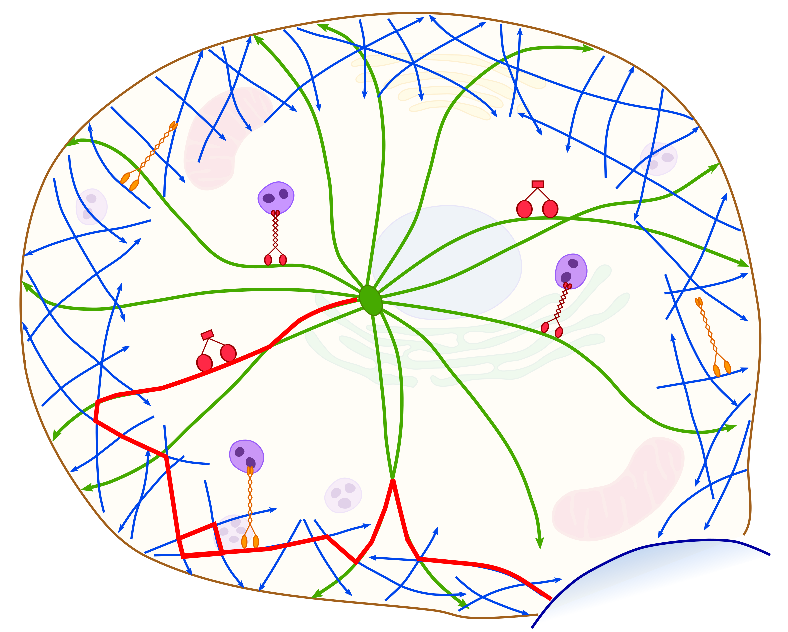
The accurate delivery of various cargoes is vital for maintaining the correct function of cells and organisms. Cargo, such as proteins, vesicles, organelles etc., is transferred to specific target destinations via molecular motor assisted transport along cytoskeletal filaments. The transport efficiency is strongly affected by the spatial organization of the cytoskeleton network, which is generally very inhomogeneous. In cells with a centrosome microtubules grow radially from the central microtubule organizing center towards the cell periphery whereas actin filaments form a dense meshwork, the actin cortex, underneath the cell membrane with a broad range of orientations. The emerging ballistic motion along filaments is frequently interrupted due to constricting intersection nodes or cycles of detachment and reattachment processes in the crowded cytoplasm. In order to investigate the efficiency of such search strategies we formulate a random velocity model with intermittent arrest states. We present a coarse grained perspective by considering the effective movement between network nodes, while discarding the single steps of individual motors at the molecular level.
Vascularized tumor growth, drug delivery, and oxygenation
Understanding the cause of cancer is a major realm within life science. Our research focuses on aspects involving the vasculature as underlying transport networks. Remodeling those networks plays an important role in being able to relate blood, nutrients and drugs transport to tumor establishment. Modern computing techniques and state of the art programming helps us to model and understand processes taking place during tumor growth which enables us to overcome the gap between in-vitro and in vivo where data is still hard to obtain.
Bystander cells enhance NK cytotoxic efficiency by reducing search time
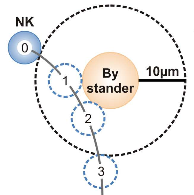
Natural killer cells play a central role in the fight of the immune system against infected or tumorigenic cells. On their search for, e.g., cancer cells the killer cells also encounter other cell types, in particular uninvolved or bystander cells. Now scientists of the SFB 1027 found out that the presence of these bystander cells does not impede the efficiency of the killers in their search for cancer cells. On the contrary, the efficiency was increased, which was totally unexpected. The reason for this boost is an accelrated migration behavior of the killers close to the bystanders, which is induced by the secretion of radical oxygen species (ROS) by the bystanders. This study is a prime example for a successful collaboration between experiment and theory and involved 4 research groups of the SFB 1027: Bin Qu (project A2), Heiko Rieger (project A3), Volkhard Helms (project C3), and Ivan Bogeski (project C4).
Spatial Organization of the Cytoskeleton enhances Cargo Delivery to Specific Target Areas on the Plasma Membrane of Spherical Cells
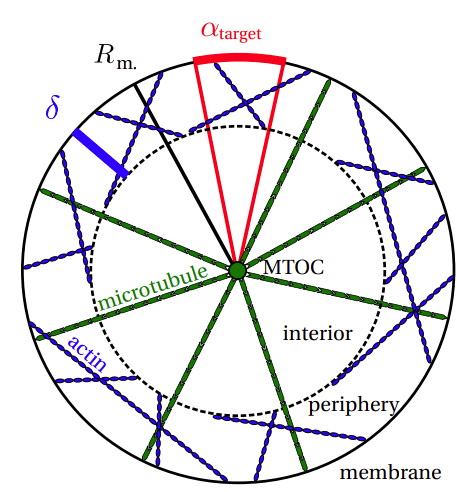
Intracellular transport is vital for the proper functioning and survival of a cell. Cargo (proteins, vesicles, organelles, etc.) is transferred from its place of creation to its target locations via molecular motor assisted transport along cytoskeletal filaments. The transport efficiency is strongly affected by the spatial organization of the cytoskeleton, which constitutes an inhomogeneous, complex network. In cells with a centrosome microtubules grow radially from the central microtubule organizing center towards the cell periphery whereas actin filaments form a dense meshwork, the actin cortex, underneath the cell membrane with a broad range of orientations. The emerging ballistic motion along filaments is frequently interrupted due to constricting intersection nodes or cycles of detachment and reattachment processes in the crowded cytoplasm. In order to investigate the efficiency of search strategies established by the cell’s specific spatial organization of the cytoskeleton we formulate a random velocity model with intermittent arrest states. With extensive computer simulations we analyze the dependence of the mean first passage times for narrow escape problems on the structural characteristics of the cytoskeleton, the motor properties and the fraction of time spent in each state. We find that an inhomogeneous architecture with a small width of the actin cortex constitutes an efficient intracellular search strategy.
Run-and-pause dynamics of cytoskeletal motor proteins
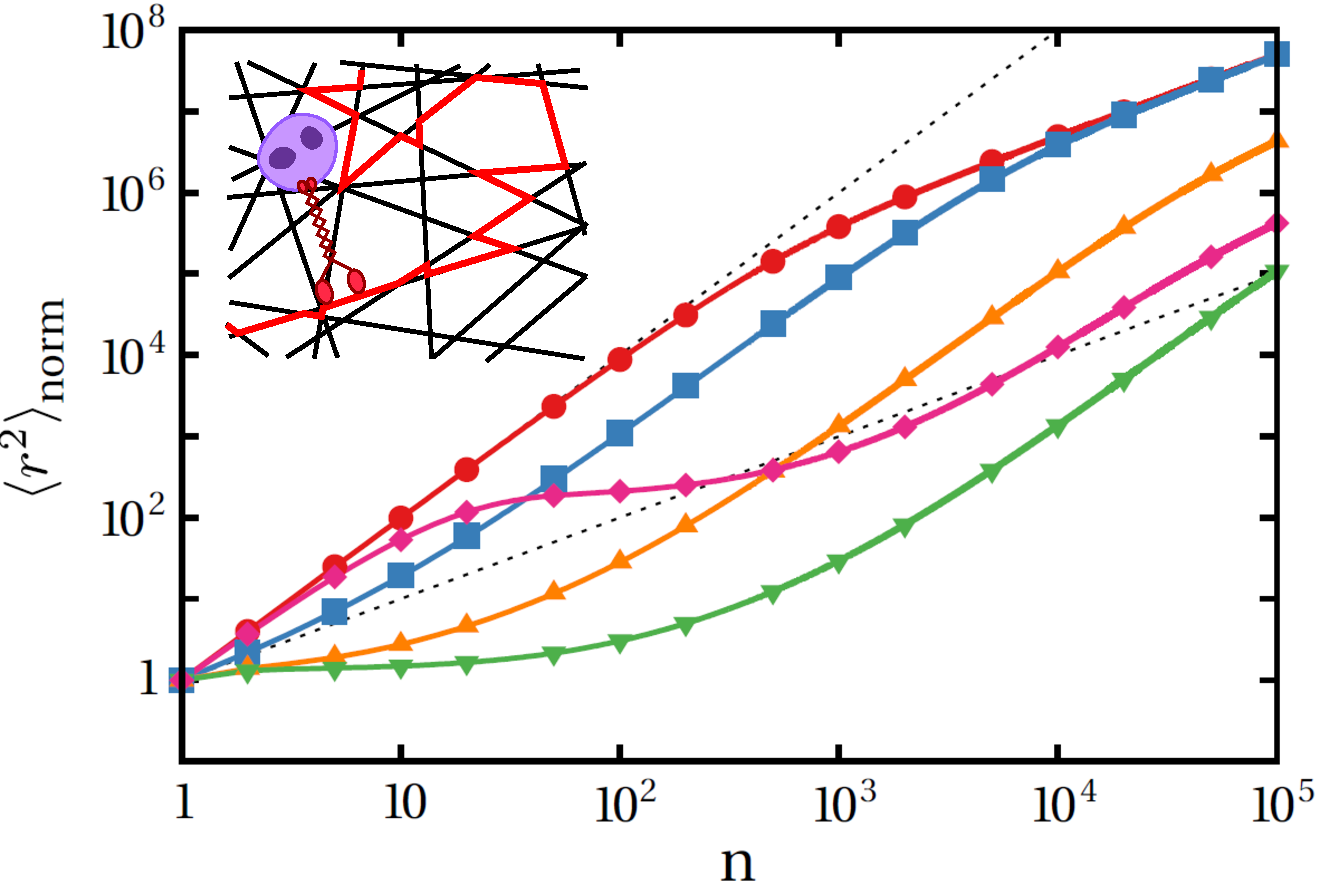
In order to maintain proper functioning, cells have to control the spatiotemporal spreading and diffusive properties of cargo particles. Cytoskeletal motor proteins are involved in major intracellular transport processes and exhibit distinct states of motility: active motion along the filaments, and pause phases in which they remain stationary for a finite time interval. The transition rates between motion and pause states are considerably affected by changes in environmental conditions which influences the diffusive properties of cargo particles. By considering the motion of molecular motors on a single filament as well as a dynamic filamentous network, we present a coarse-grained random walk description and an analytical model for the dynamics of self-propelled particles which undergo frequent pause phases.
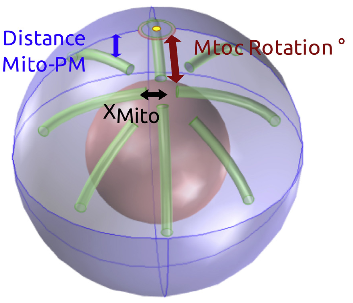
Cytoskeleton rotation relocates mitochondria to the immunological synapse and increases calcium signals
Thiol dependent intramolecular locking of Orai1 channels
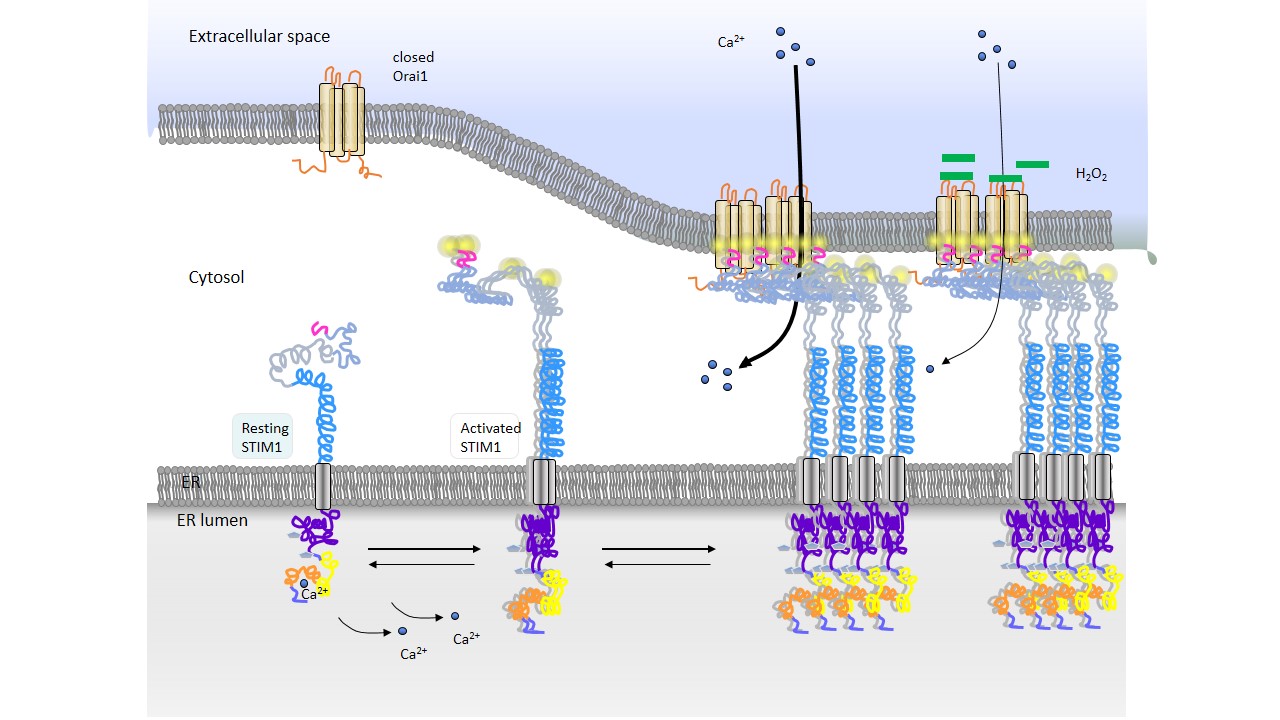
Differential Calcium Ca2+ signals drive alternate cell fates such as proliferation, apoptosis or tolerance. In immune cells one major entry pathway for Ca2+ into the cells are store-operated Ca2+ channels. In response to store depletion ER-resident Ca2+ sensor molecules STIM translocate to regions near the plasma membrane and interact with Orai1 proteins. These form the major ion conduction units mediating the Ca2+ release activated ICRAC (CRAC) current. Reactive oxygen species (ROS) interacting with cysteine residues can alter protein function. Pretreatment of Orai1 with the oxidant H2O2 reduces ICRAC . Our results demonstrate a novel mechanistic model for ROS-mediated inhibition of Orai1 and identify a candidate residue for pharmaceutical intervention.
A calcium-redox feedback loop controls human monocyte immune responses: The role of ORAI Ca2+ channels
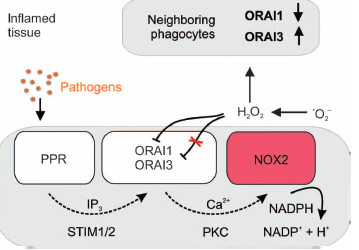
Monocytes are central effector cells of the innate immune system. As professional phagocytes, they are involved in eliminating pathogens and in activating other immune cells, such as T cells. After recognizing pathogens, Ca2+ is mobilized and a rapid production of large amounts of reactive oxygen species (ROS) is initialized (Nox2–mediated “oxidative burst”). We showed that Orai channels control store-operated Ca2+ entry, ROS production, and bacterial killing in primary human monocytes. The physiological functions of human monocytes depend on both Ca2+ and redox signals [1]. Therefore, these phagocytic cells represent an ideal cellular system for examining the complex interplay between Ca2+ and ROS. With the help of a mathematical model that predicts additional features of the Ca2+ -redox interplay we identified the Orai-Nox2 feedback loop as determinant of monocyte immune response.
Co-Chaperones of the mammalian endoplasmic reticulum
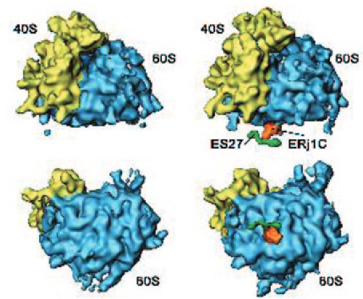
In mammalian cells, the rough endoplasmic reticulum or ER plays a central role in the biogenesis of most extracellular plus many organellar proteins and in cellular calcium homeostasis. Therefore, this organelle comprises molecular chaperones that are involved in import, folding/assembly, export, and degradation of polypeptides in millimolar concentrations. In addition, there are calcium channels/pumps and signal transduction components present in the ER membrane that affect and are affected by these processes. The ER lumenal Hsp70, termed immunoglobulin-heavy chain binding protein or BiP, is the central player in all these activities and involves up to seven different co-chaperones, i.e. ER-membrane integrated as well as ER-lumenal Hsp40s, which are termed ERj or ERdj, and two nucleotide exchange factors.
Interplay of channels, pumps and organelle location in calcium microdomain formation
To analyze the influence of Ca
2+
microdomains on the global
cytosolic
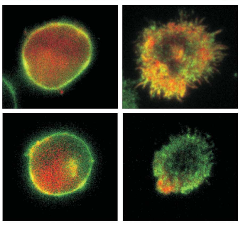
Docking of Lytic Granules at the Immunological Synaps in Human CTL Requires Vti1b-Dependent Paiiring with CD3 Endosomes
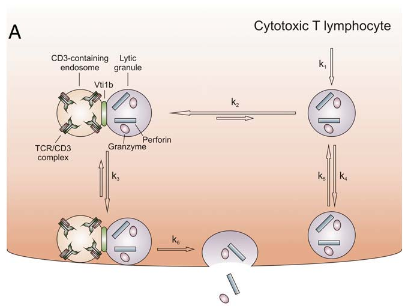
Lytic granule (LG)-mediated apoptosis is the main mechanism by which CTL kill virus-infected and tumorigenic target cells. CTL form a tight junction with the target cells, which is called the immunological synapse (IS). To avoid unwanted killing of neighboring cells, exocytosis of lytic granules (LG) is tightly controlled and restricted to the IS. In this study, we show that in activated human primary CD8+ T cells, docking of LG at the IS requires tethering LG with CD3-containing endosomes (CD3-endo). Combining total internal reflection fluorescence microscopy and fast deconvolution microscopy (both in living cells) with confocal microscopy (in fixed cells), we found that LG and CD3-endo tether and are cotransported to the IS. Paired but not single LG are accumulated at the IS. The dwell time of LG at the IS is substantially enhanced by tethering with CD3-endo, resulting in a preferential release of paired LG over single LG. The SNARE protein Vti1b is required for tethering of LG and CD3-endo. Downregulation of Vti1b reduces tethering of LG with CD3-endo. This leads to an impaired accumulation and docking of LG at the IS and a reduction of target cell killing. Therefore, Vti1b-dependent tethering of LG and CD3-endo determines accumulation, docking, and efficient lytic granule secretion at the IS.
Calcium microdomains at the immunological synapse: how ORAI channels, mitochondria and calcium pumps generate local calcium signals for efficient T-cell activation
Cell polarization enables restriction of signalling into micro- domains. Polarization of lymphocytes following formation of a mature immunological synapse (IS) is essential for calcium-dependent T-cell activation. Here, we analyse calcium microdomains at the IS with total internal reflection fluorescence microscopy. We find that the subplasmalemmal calcium signal following IS formation is sufficiently low to prevent calcium-dependent inactivation of ORAI channels. This is achieved by localizing mitochondria close to ORAI channels. Furthermore, we find that plasma membrane calcium ATPases (PMCAs) are re-distributed into areas beneath mitochondria, which pre vented PMCA up-modulation and decreased calcium export locally. This nano-scale distribution—only induced following IS formation—maximizes the efficiency of calcium influx through ORAI channels while it decreases calcium clearance by PMCA, resulting in a more sustained NFAT activity and subsequent activation of T cells.
Legal notice (Impressum) Privacy policy



