AG — Prof. Heiko Rieger — Statistical Physics
Vascularized tumor growth, drug delivery and oxygenation
( source could be found here )
News
An image from our recent publication (Tumorcode. Eur. Phys. J. E 41, 55 (2018)) covers the April issue of EPJE and got featured on “Springer Nature Grand Challenges”. We agree with the editor that understanding the complex interplay between vasculature and tumors is a grand challenge for global health.
Our source code is maintained on github.
Introduction
"Physics of Cancer" has recently become a major interdisciplinary research field. The reason is, even after decades of research, since the declaration of the ``war against cancer'' in 1971 in the US, cancer is a leading cause of death worldwide and is an ever-growing problem in the aging population. Altogether, average patient survival times have improved significantly only for a few types of cancer. Today, drug resistance and obstacles to delivery are still major challenges to overcome.
Blood vessel networks of solid tumors such as melanomas, brain-or breast tumors, exhibit abnormalities that make them much different from normal vasculatures. For instance, (micro) vascular density (MVD) is not uniform as in normal capillary beds. Instead, tumor centers typically exhibit lower MVD whereas dense capillary excrescence permeates its periphery. Remaining vessels of the interior may consist of just relatively few vascular threads with cuffs viable cells around them. Distal from such vessels, necrotic regions emerge due to the lack of nutrients. The organization into arterioles, capillaries and veins is lost, instead tumor vessels are chaotically connected, dilated and tortuous. Tumor vessels are also leaky, i.e. they exhibit gaps in their walls through which the blood stream is in direct contact with tumor cells and the extracellular matrix (fibers and liquid in-between cells).
"This heterogeneity hinders the delivery of blood-borne therapeutics to all cancer cells and leads to acutely and chronically hypoxic and acidic regions in tumors. These conditions reduce the effectiveness of radiation and some chemotherapeutic agents and select for cancer cells that are more aggressive metastatic, and resistant to various therapies" [Jain (2001) PNAS]
Recent therapeutic strategies target the vascular network of tumors in order to either reduce wall permeability and improve blood flow, thereby improving drug delivery, or on the other hand, to completely deprive tumor cell of nutrients. The first approach is known as vascular normalization. However the mechanistic understanding of vascular network formation and drug delivery is still lacking. Moreover there is no general theory of cancer with predictive power.
State of the art theoretical modeling
The aim of our research is to shed light on the physical determinants of tumor growth and tumor blood vessel network formation. Moreover, we study the interrelation between vascular network morphology, blood flow, interstitial fluid flow, oxygenation and drug delivery.
To this end, we formulate spatiotemporal theoretical models of such growing tumors and analyze their predictions. Our model tissue comprises a growing multi-cellular tumor spheroid and a dynamic vasculature, represented by a network of interconnected blood perfused pipes. Concentration distribution fields of oxygen and growthfactors, of which the latter are signaling substances, mediate interactions between tissue and vasculature. For further reading see also the reviews[2, 3, 5, 7, 11]
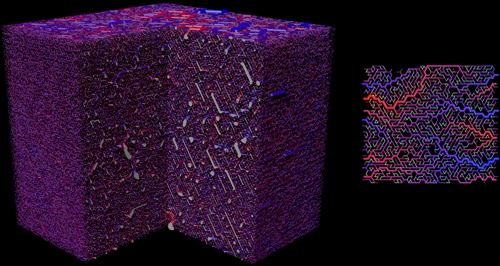
Visualization of the initial vasculature for normal tissue. Left: View on a three dimensional system. A quadrant was cut out. The lateral length is approximately 8 mm. Right: A small two dimensional example. Vessels are color coded by blood pressure, where red is highest (arteries) and blue is lowest (veins). The construction algorithm grows and shrinks arterial and venous trees depending on blood flow. Capillaries, in light green here, connect both types of trees.
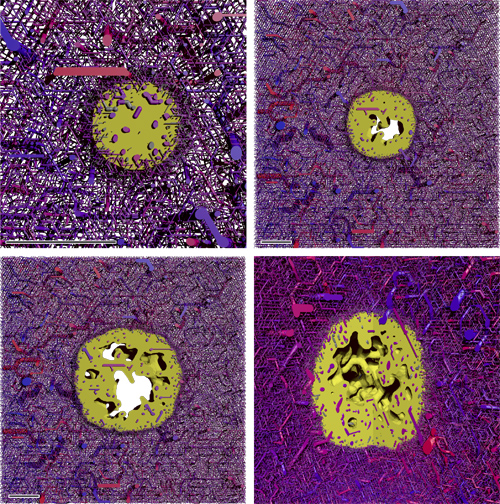
Snapshots from the simulation of a growing tumor. (A) to (C) depict 400 micron thick slices through the origin. The scale bar indicates 1 mm. (A) is a close-up. (B) and (C) have the same scale. The snapshots are taken after 100 h, 400 h, and 700 h. D shows the same time as (C) from a different point of view where a quadrant was cut out. The boundary to the viable tumor mass is rendered as solid yellow surface. Necrotic regions appear as void spaces within the tumor. The blood vessel network is rendered as collection of cylinders, color coded by blood pressure. Red is high, and blue is low.
Angiogenesis is a hallmark of cancer. It is the process of growing new blood vessels from the existing vasculature which a tumor uses to procure additional nutrients for growth. Otherwise a small nucleus is limited to the typical size of one millimeter due to the finite diffusion range of nutrients. Original vessels and neovasculature are co-opted by the tumor when the invasive edge of the spheroid expands past them. In the tumor interior, a dramatic degradation of vascular walls and regression takes place. Moreover vascular growth is switched from angiogenesis to circumferential growth. Thus, the vascular network of the host is continuously transformed near the invasive edge into the typical chaotic structure of tumor vasculatures.
Our model 12 incorporated, to the best of our knowledge, for the first time vascular remodeling processes that lead to the prediction of realistic tumor vascular morphology: blood-flow, angiogenesis, co-option, dilation and regression and collapse. In practice this means that, vessel segments are dynamically created and deleted. Also properties associated with them change over time such as their radius.
Blood flow, or rather shear stress exerted on vessels walls, is sensed by endothelial cells of the wall. Physiological levels of shear stress are required for the maintenance of the vasculature. Otherwise blood vessels tend to regress. It was shown that this correlation between shear stress and regression leads to realistic predictions of vascular morphology [13, 10, 9].
A synthetic arterio-venous initial vasculature used in state of the art research resembles the situation in patients [9, 7b, 6, 4]. These models predict formation of arteriovenous shortcuts by dilated vessels and that tumor vessels are more likely to survive in areas where vessels with high blood pressure difference are close to each other in the initial network.
Interstitial fluid flow and drug transport
We performed simulations of advection of an intra-vascular tracer. However, simulated tumors are rapidly perfused, saturating essentially the entire vasculature with tracer within minutes. For such rapidly perfused tumors, a blood-borne delivery problem of drug does not appear to exist. This does not mean that there are no other barriers to drug delivery - such as poor tissue penetration of drugs.
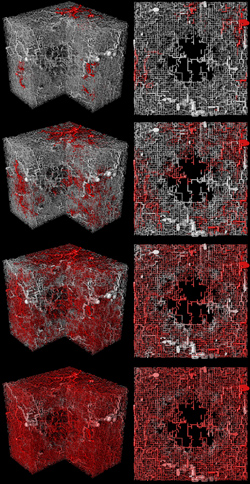
Visualization of intravascular tracer flow. The color code displays the concentration of the tracer agent, where red is the maximal concentration at the inflow nodes. The time progresses from top to bottom to ca 5 s after the start of an infusion of tracer at constant concentration.
In subsequent work, we performed an extensive study of interstitial fluid flow (IFF) and drug delivery through the interstitial space [6]. Interstitial fluid (IF) is the liquid which leaks through gaps in the capillary walls, flows through the empty spaces in-between tissue cells to be absorbed into lymphatic channels and conveyed back into the blood stream. Leakiness of tumor vascular walls and the absence of functional lymphatics lead to a drastically elevated interstitial fluid pressure (IFP). The resulting IFP gradient drives IF from the tumor interior into surrounding normal tissue.
We model IFF as flow through a homogeneous porous medium subject to Darcy´s law with appropriate source and sink terms reflecting the fluxes from vessel network and lymphatics. It is often stated that an elevated IFP is a barrier to drug delivery, preventing extravasation. However the flow of IF should rather be regarded as current through a series of resistors. In this case, the flow velocity through the system increases with the various permeabilities. Therefore the IF flow increases with wall permeability until the loss of blood plasma becomes significant enough to impede blood flow.
Drug concentration distributions in tissue were computed by solution of a diffusion-advection-reaction equations, using the pre-calculated velocity field of the IF. Predicted local doses are predominantly determined by the vessel density. Even for heavy, non-diffusing agents, convection leads to significant doses in most areas throughout the whole tumor. However, as dictated by the random arrangement of tumor vessels and the resultant IF velocity field, small patches of viable tumor mass receives comparably negligible doses. This suggests that cancer recurrence is a likely to occur after a therapy utilizing macro-molecules or nano-scale particles.
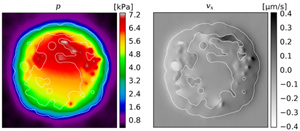
Left: The local IFP. Right: The x-component of the local IF velocity. The plots show a slice through simulation results for a ca 4 mm diameter. The white outline delineates the viable tumor mass. Note that we assumed an increased conductivity for necrotic regions, hence the discontinuous velocity.
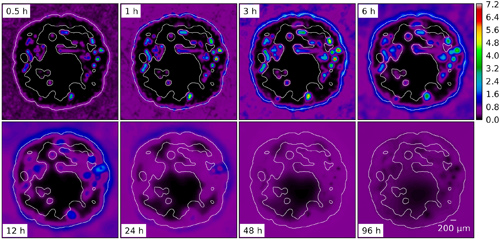
Snapshots from the evolution of an agent concentration distribution in the interstitial space. Here we used parameters for Doxorubicin (ca. 540 g/mol) which leads to a diffusion dominated system except for the periphery where convective fluxes are of similar magnitudes. After the initial injection the distribution disperses gradually and the agent is slowly re-absorbed into the "clean" vasculature.
Tumor Oxygenation
The goal of this project is to qualitatively explain published data of optical mammography, which were obtained from 87 breast cancer patients. The data generally show average hemoglobin concentration to be higher in tumors versus host tissue. However the blood oxygen saturation can be above or below normal.
To this end we developed a computational model with which we determine concentration distributions of oxygen in vascular networks [4]. The amount of oxygen lost by by diffusion into tissue must be properly accounted for. For our problem it is not sufficient to assume a constant blood oxygen concentration, or a constant hemoglobin saturation. Therefore, spatially varying distributions of intravascular and extravascular oxygen levels must be computed simultaneously. On the basis of this model we consider oxygenation in synthetic tumor configurations grown in different initial vascular networks emulating a cohort of patients.
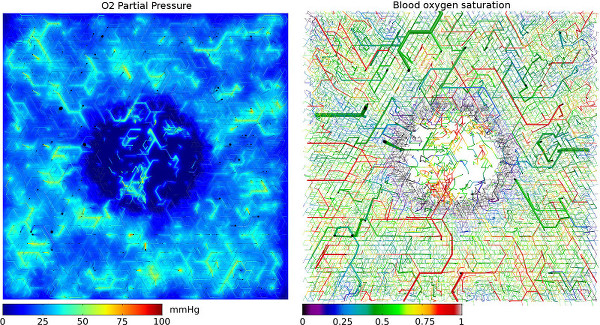
Simulated oxygenation in an synthetic vascular network. The displayed section is a slice through the center of the three-dimensional simulation box. Left side shows oxygen partial pressure in tissue, as well. Blood vessels, represented by cylinders, are truncated 100 micrometer above and below the central plane.
References
| Tumorcode - A framework to simulate vascularized tumors |
|
| The European Physical Journal E 41, 55 (2018) | [bioRxiv], [pdf], [ Springer Nature Grand Challenges], [github], [Highlight- EPJE] |
| Computer Simulations of the Tumor Vasculature: Applications to Interstitial Fluid Flow, Drug Delivery, and Oxygen Supply |
|
| "Systems Biology of the Tumor Environment: Quantitative Modeling and Simulation", ed. K. Rejniak, Springer (2016) | [pdf] |
| Physics of the tumor vasculature: Theory and experiment |
|
| Eur. Phys. J. Plus 131, 31 (2016) | [pdf] |
| Computational model for tumor oxygenation applied to clinical data on breast tumor hemoglobin concentrations suggests vascular dilatation and compression |
|
| PLOS ONE 11, e0161267 (2016) | [pdf] |
| Integrative models of vascular remodeling during tumor growth |
|
| Invited Review, WIREs Systems Biology and Medicine 7, 113 (2015) | [pdf] |
| Interstitial fluid flow and drug delivery in vascularized tumors: A computational model |
|
| PLOS ONE 8, e70395 (2013) | [PONE], [pdf] |
| Blood vessel network remodelling during tumor growth |
|
| Modeling Tumor vasculature: Molecular, Cellular, and Tissue Level Aspects and Implications. ed.: Trachette Jackson (2010) | [pdf] |
| Physical determinants of vascular network remodeling during tumor growth |
|
| Europ. Phys. J. E 33, 149 (2010) | [pdf], [doi] |
| Vascular remodelling of an arterio-venous blood vessel network during solid tumor growth |
|
| J. Theor. Biol. 259, 405 (2009) | [DOI], [q-bio], [pdf] |
| Emergent vascular network inhomogenities and resulting blood flow patterns in a growing tumor |
|
| J. Theor. Biol. 250, 257 (2008) | [pdf] |
| Morphology of tumor vasculature: A theoretical model |
|
| Proceedings ECMTB '05, Mathematical Modeling of Biological Systems, Volume I., A. Deutsch, L. Brush, H. Byrne, G. de Vries and H.-P. Herzel (eds). Birkhäuser, Boston, 231-244 (2007) | [pdf] |
| Vascular network remodeling via vessel cooption, regression and growth in tumors |
|
| J. Theor. Biol. 241, 903 (2006) | [ q-bio], [pdf ] |
| Flow correlated percolation during vascular network formation in tumors transition |
|
| Phys. Rev. Lett. 96, 058104 (2006) | [ q-bio], [pdf] |
Figures
Presentations and Media
- Presentation file: Dynamical remodeling of the vascular network during tumor growth (ppt)
- Presentation file: Vascular network remodeling via vessel cooption, regression and growth in tumors (pdf)
- Video: 3d model of tumor-induced angiogenesis (avi)
- Video: Interstitial concentration of diffusion dominated agent (avi)
- Video: Interstitial concentration of a non-diffusing agent (avi)
- Video: 2d model of tumor-induced angiogenesis (avi)
- Video: Model for drugflow through tumor vasculature(avi)
- Video: Tumor induced angiogenesis in arteriovenous network (XVid MPEG4 avi)
Legal notice (Impressum) Privacy policy




